Research Programme: ENERGY
ENERGY: DeciphEriNg tumour mEtabolic cRoss-talks for new diaGnostic and therapeutic opportunitY
Coordinators

MD, PhD, Head of Pathology Department (CHU)

Head of the « Molecular oncogenesis » team (IRCM)
ENERGY Programme relies on a consortium of medical and scientific leaders with international recognition in metabolism, epigenetics and data modeling aimed at developing innovative therapeutic strategies based on the improved understanding of the metabolic alterations of cancer cells.
The goal of this Axis is to decipher how metabolic changes in cancer cells modulate gene expression programmes, thereby influencing tumour evolution. By combining our unique expertise in the fields of metabolism and chromatin/RNA modifications, our objective is to further understand the associations between several key metabolites and the epigenome/epitranscriptome in order to develop new combined therapies.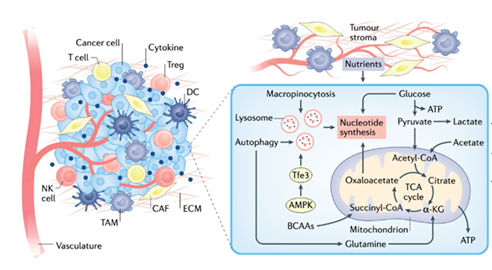
In this Axis, researchers will address how perturbations of iron, pyruvate, amino-acid and lipid metabolism fuel intra-tumoral heterogeneity, a major determinant of drug resistance and tumour relapse. Notably, the role of nutrient and oxygen availability in the spatial organization of the tumour ecosystem will be studied. teams specialising in tumour ecosystem analysis will investigate new mechanisms by which different populations of cancer cells communicate with surrounding stromal and immune cells and exchange metabolites to influence cancer progression. Local experts and international collaborators in cancer metabolism, immunology, computation-based pathology (epathology) and clinical/molecular imaging will actively collaborate to translate the results of this axis into new therapeutic strategies.
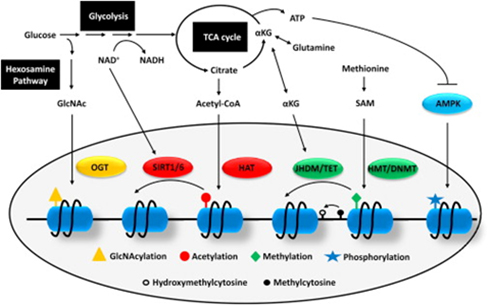
In this axis, the metabolic effects of tumour cells on distant organs will be studied in order to identify the mechanisms by which changes in the metabolic state of the host influence tumour progression and response to drugs. Projects in this axis should shed light on the role of systemic metabolic changes in cancer progression and characterise molecular mechanisms that can be manipulated by drugs as well as non-pharmacological interventions.